Imfinzi Stage Iii Lung Cancer
cancer imfinzi wallpaperSince the first approval in February 2018 more than 20000 patients in this setting have been treated with Imfinzi. For adults with unresectable Stage 3 non-small cell lung cancer NSCLC whose disease has not progressed following concurrent chemoradiation therapy CRT.

The US Food and Drug Administration FDA has approved Imfinzi durvalumab for people with stage III 3 non-small cell lung cancer who are not able to be treated with surgery to remove their tumor and whose cancer has not gotten worse after they received chemotherapy along with radiation chemoradiation.
Imfinzi stage iii lung cancer. José Baselga Executive Vice President Oncology RD said. Food and Drug Administration today approved Imfinzi durvalumab for the treatment of patients with stage III non-small cell lung cancer NSCLC whose tumors are not able to be surgically. Approximately one third of patients with nonsmall-cell lung cancer NSCLC have stage III locally advanced disease at diagnosis.
For one it could limit one of Imfinzis expansion opportunities outside the safe harbor indication it bears in stage 3 non-small cell lung cancer by far Imfinzis largest source. A 1500 mg fixed dose administered every 4 weeks for the treatment of unresectable stage III non-small cell lung cancer NSCLC after chemoradiation therapy CRT and previously treated advanced bladder cancer according to AstraZeneca the developer of the agent. IMFINZI is the first and only immunotherapy for people with unresectable Stage 3 non-small cell lung cancer NSCLC whose disease has not progressed following concurrent platinum-based chemoradiation therapy CRT.
IMFINZI durvalumab is a prescription medicine used to treat adults with a type of lung cancer called non-small cell lung cancer NSCLC. The graphic above is simplified to help you better understand the stage of your lung cancer and should not take the place of talking with your treatment team. Adults with unresectable Stage III non-small cell lung cancer whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.
Jonathan Goldman MD explains IMFINZI is the first immunotherapy to show both significant survival benefit and improved durable responses in extensive-stage small cell lung cancer. IMFINZI may be used when your NSCLC has not spread outside your chest cannot be removed by surgery and has responded or stabilized with initial treatment with chemotherapy that contains platinum given at the same time as radiation therapy. The only approved immunotherapy to help people live longer following CRT.
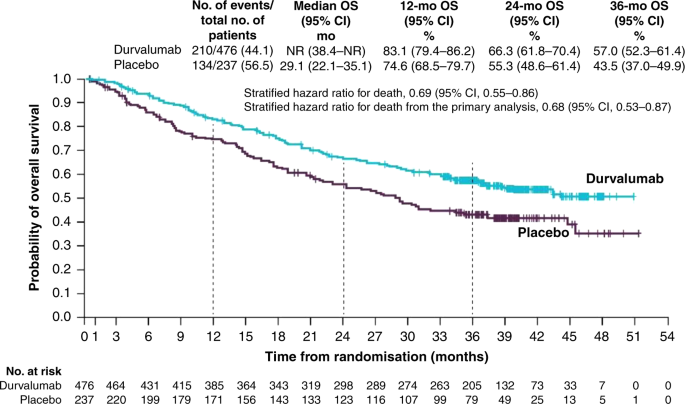
These unprecedented four-year results reinforce Imfinzi as the established standard of care in unresectable Stage III non-small cell lung cancer and set a new survival benchmark in a setting where cure is the treatment goal. Imfinzi is approved for the treatment of unresectable Stage III non-small cell lung cancer in more than 45 countries including the US EU and Japan based on the Phase III PACIFIC trial. IMFINZI may be used when your NSCLC has not spread outside your chest cannot be removed by surgery and has responded or stabilized with initial treatment with chemotherapy that contains platinum given at the same time as radiation therapy.
In a clinical trial 66 of people taking IMFINZI were alive compared with 56 of those taking placebo no medicine at 2 years. Historically patients with a good performance status and stage III locally advanced unresectable nonsmall-cell lung cancer NSCLC have been treated with platinum-based doublet. The FDA has approved an additional dosing option of durvalumab Imfinzi.
1 The standard of care for patients with a good performance. Exploratory subgroup analyses from another Phase 3 CASPIAN evaluating Imfinzi chemo in patients with extensive-stage small cell lung cancer SCLC showed that more than 3x 17 vs. IMFINZI durvalumab is a prescription medicine used to treat adults with a type of lung cancer called non-small cell lung cancer NSCLC.
In combination with etoposide and either carboplatin or cisplatin as first-line treatment for adults with extensive-stage small cell lung cancer. Imfinzi is approved in the curative-intent setting of unresectable Stage III non-small cell lung cancer NSCLC after chemoradiation therapy in the EU US Japan China and many other countries. In the Phase III CASPIAN trial IMFINZI at a fixed convenient dose improved survival with either a cisplatin or.
Additionally it is approved in the EU US Japan and many other countries for the treatment of extensive-stage small cell lung cancer SCLC. If you have questions about Stage 3 non-small cell lung cancer ask your doctor about IMFINZI download the Doctor Discussion Guide and take it to your next appointment.

